Light it up: Battery particles tell the true story of a battery's charge
Light it up: Battery particles tell the true story of a battery's charge
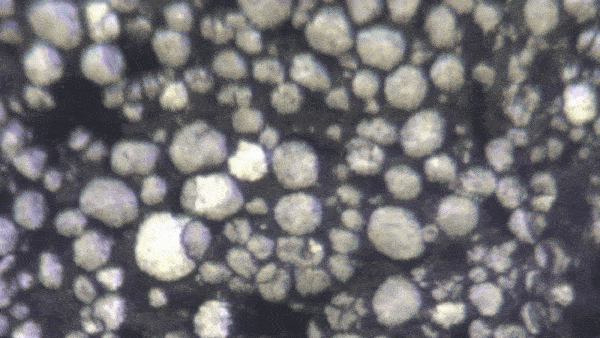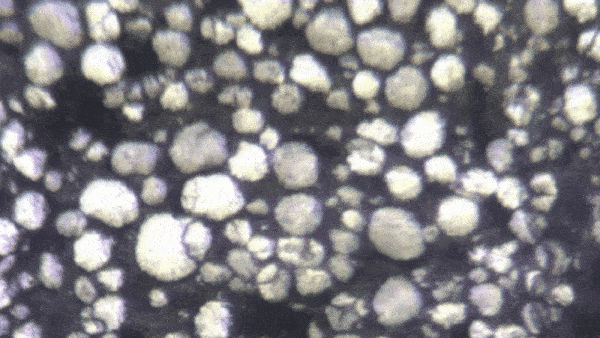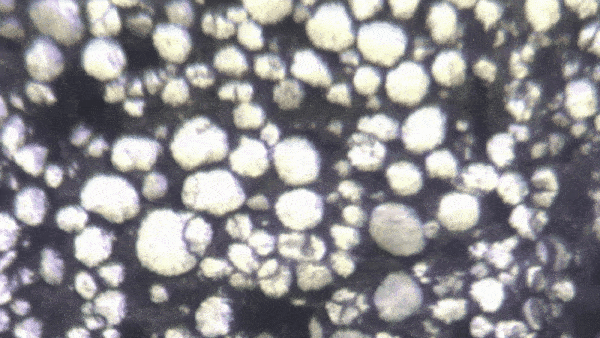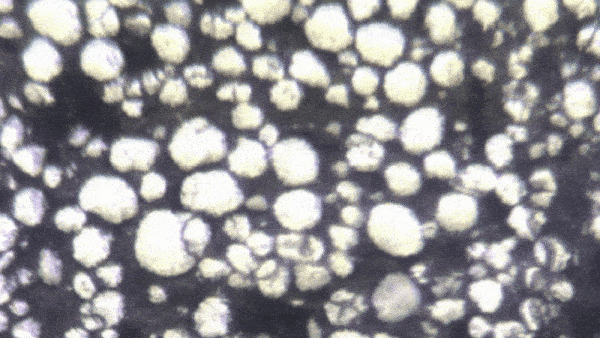
“Lithium-ion batteries — in fact, all batteries — function because of millions of chemical interactions happening at the particle level,” said Kejie Zhao, professor of mechanical engineering. “Characterizing them becomes a mechanical and electrochemical problem.”
In Zhao’s lab, they use many tools to bridge this gap between mechanics and electrochemistry to create better batteries. One of these tools is a simple RGB camera.
“It’s only been recently discovered that individual particles in a battery’s electrode actually appear brighter as they charge,” Zhao said. “Our breakthrough is that we look at hundreds of particles at a time, and can use their brightness levels to determine how evenly the charge is distributed through the electrode.”
This research has been published in Proceedings of the National Academy of Sciences.
The experiment began with a lithium-ion coin cell battery in a glove box filled with inert gas (lithium is volatile when exposed to the open air). Zhao's team focused a simple optical microscope onto a group of 100 to 1,000 individual particles. They slowly charged the battery and recorded time-lapse video of the same group of particles over several hours. By analyzing the brightness level of these individual particles, they could reconstruct an extremely precise spatial model of how evenly the battery is charged.
“The amazing part about this process is that you don’t need high-powered tools — just a simple optical microscope and camera,” Zhao said. “It doesn’t even need to be in focus; the brightness levels consistently deliver accurate data either way.”
With image processing and data analysis, the team extracted valuable data about the construction and operation of these batteries. “Right now, the only way to quality control for a battery’s particles is to examine them in the factory,” Zhao said. “But by observing optically how they charge over time, we get a more accurate picture. We’ve established that there is a direct mathematical correlation between the optical brightness of particles and the overall state of charge of the battery.”
And that’s important, because a battery’s charging and discharging behavior relies on its heterogeneity, or how evenly distributed particles are throughout the electrode. If charge is concentrated in one place, the battery is more likely to degrade, fail, or even burn catastrophically. “Even at the particle level, clusters of charge can lead to local defects, which can lead to degraded performance and eventually thermal runaway,” Zhao said.
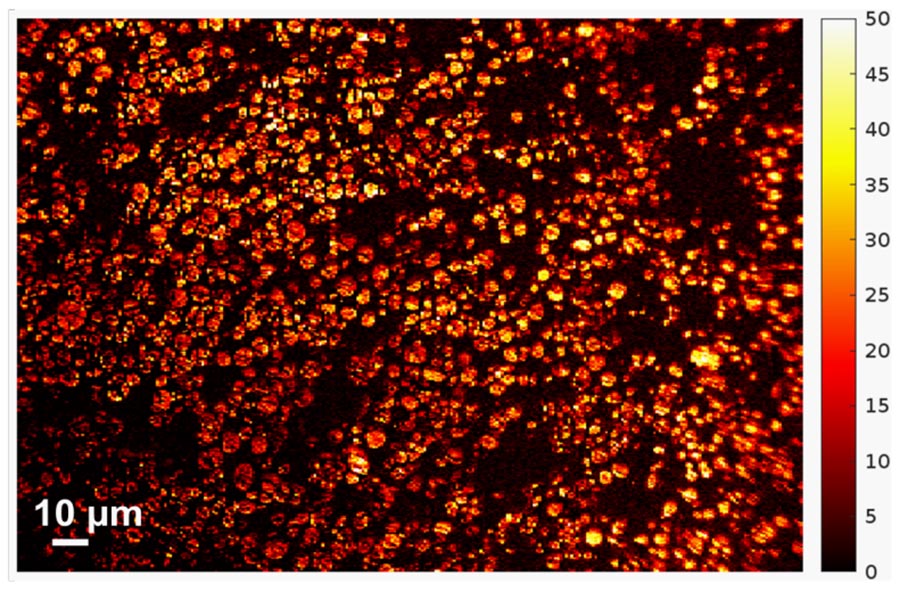
While Zhao’s experiments focused specifically on lithium nickel manganese cobalt oxides (NMC), he said that this optical process has been proven to work for many electrode materials — lithium cobalt oxide, graphite, and others — because of the change of electrical conductivity upon charging and discharging. In other words, this characterization process can be used for any type of battery formulation in the future.
“Batteries have always been difficult to diagnose,” he said. “Seeing them behave like this, in an active charging or discharging state, offers so much more information than analyzing them in a static state. We’ve proven the theoretical foundation, and now we can use optical microscopes with confidence to analyze today’s batteries and establish the science for future battery technologies.”
This research is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under award number DE-SC0024064; and by the National Science Foundation under award number CBET-2349666.
Source: Kejie Zhao, [email protected]
Writer: Jared Pike, [email protected], 765-496-0374
On the Scale of Heterogeneity in Composite Electrodes of Batteries
Aishwary Shrivastava, Nikhil Sharma, Xiaomei He, Yijin Liu, Kejie Zhao
https://doi.org/10.1073/pnas.2520136122
ABSTRACT: An electrode in cylindrical or pouch cell batteries contains millions of active particles embedded in a conductive network. Battery performance, such as voltage, capacity, and cyclic efficiency, is a collective response of the particle network. We use optical microscopy to measure the local, heterogeneous state of charge of individual particles upon charging and discharging. The optical reflectivity is proportional to Li composition in the ternary oxide LiNixMnyCozO2 (NMC) cathode. Through clustering analysis, we determine the scale of heterogeneity where a representative volume in the composite electrode contains 100-1000 particles. The heterogeneous activity in the particle network can be described by Weibull defect population at the particle interface with the conductive matrix.
